Newsletter February 2026
To all shareholders

Auxesis Pharma AB (publ.) celebrates
10-year anniversary in 2026
Auxesis Pharma has used much of the Christmas and New Year holidays to prepare for the start of 2026. Our focus is to initiate the clinical studies.
Auxesis Pharma AB celebrates its 10-year anniversary in 2026. The early years began with basic research on acetylsalicylic acid, followed by different solution approaches. In recent years, laboratory work has focused on achieving a stable, liquid ASA solution for a new medicine. Last year, we reached a milestone with a stable formulation showing excellent penetration, which we are now taking forward into clinical trials.
A hallmark of Auxesis Pharma AB is perseverance and reliability—building market and customer confidence that we deliver the right and effective formulation for an OTC medicine that removes pain.

EMA SME status approved:
For the fifth time, Auxesis Pharma has had its EMA/SME status for 2026 approved by the European Medicines Agency. This is a very important tool for Auxesis Pharma as we move closer to launches.
Clinical studies with ASA.P

We have now completed the formulation preparations for the finished medicine and in the coming weeks will submit the application to the Swedish Medical Products Agency to start the clinical trials. The application to the Ethics Review Authority has already been submitted.
The clinical trials will be completed during the summer. The results will then be compiled into protocols used for the marketing authorization application.
We collaborate daily with our formulation team in Lund, our regulatory experts who draft the protocols in Uppsala, and the CRO conducting the clinical studies on healthy volunteers—also in Uppsala. A tightly knit team with deep expertise in topical solutions and drug studies.
Because Auxesis Pharma is developing a liquid formulation of acetylsalicylic acid, we have also developed a completely new method for how clinical studies should be performed in the clinic—ensuring both quality and simplicity in the trial itself.
New products
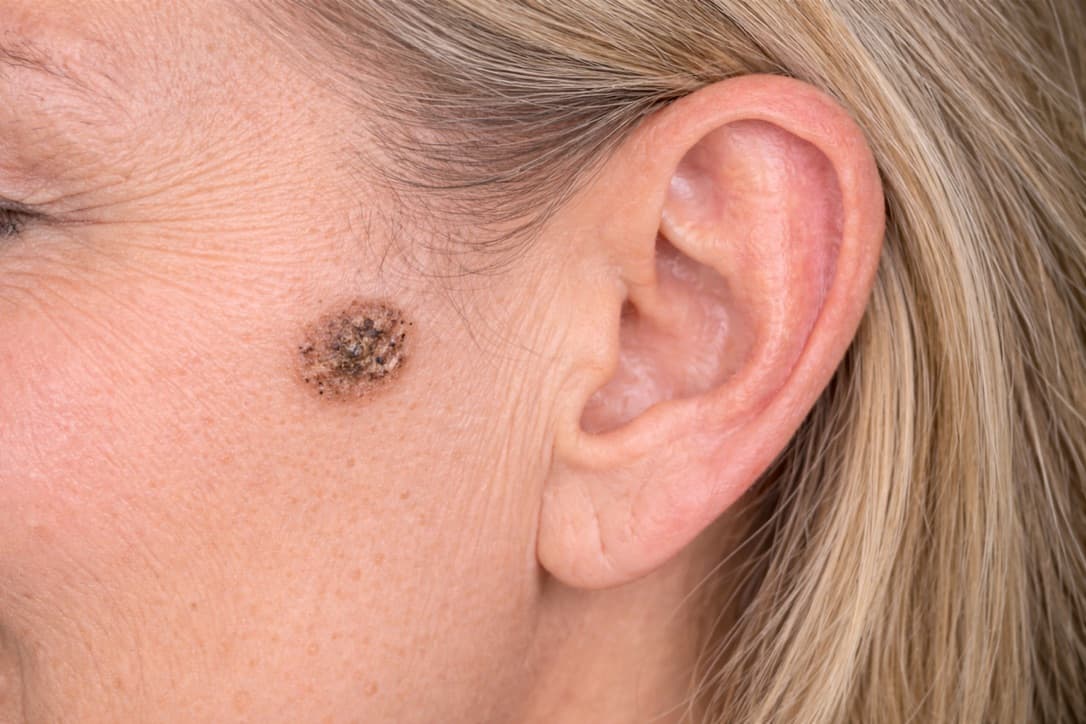
During our journey to develop a medicinal solution for liquid acetylsalicylic acid—ASA.P—which is now moving into clinical studies, we are discovering new opportunities to treat conditions and diseases related to the skin. This ranges from removing skin spots to treating wounds and burns at different levels.
It is an exciting development to both identify and practically develop solutions for new medicines that can bring significant societal benefit to patients with skin conditions or wound injuries.
We have already initiated tests with different formulations, both within our own team and together with medical experts across several fields.
First up is a prospective medicine for seborrheic keratosis (age warts)—a very common, benign epidermal tumor. 50% of adults over 50 have one or more of these keratoses, often cosmetically bothersome. They occur in all populations. Family predisposition, UV exposure, and age contribute. We are now developing a medicine that removes these spots completely. Treatment to date has included freezing (cryotherapy), curettage, or laser treatment.
In the next newsletter, we will describe in more detail the different steps in Auxesis Pharma’s future drug development—some of which may be launched in the near term.
Our new website is now finished and published.
In collaboration with Holycomms AB and EnterCode KB, Auxesis has completed an impressive renewal of the website—resulting in a more modern look with a new logo, new fonts, and more informative content.